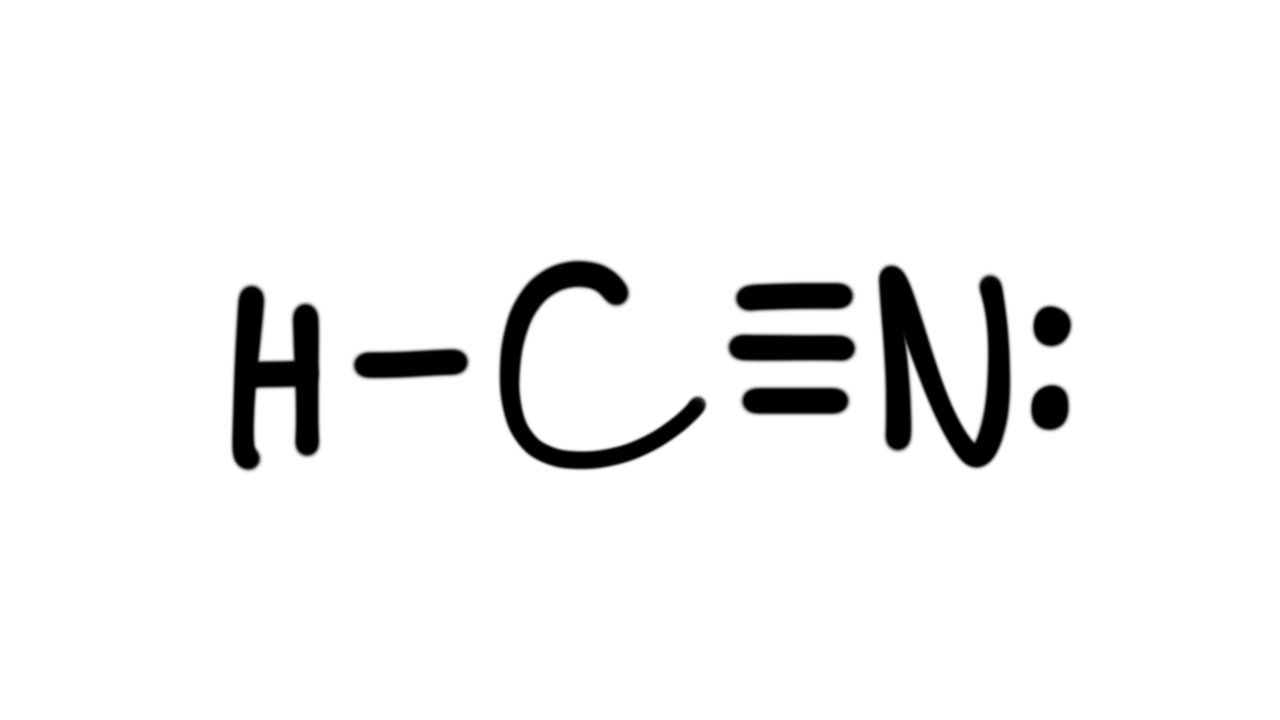Draw The Lewis Structure Of Hcn
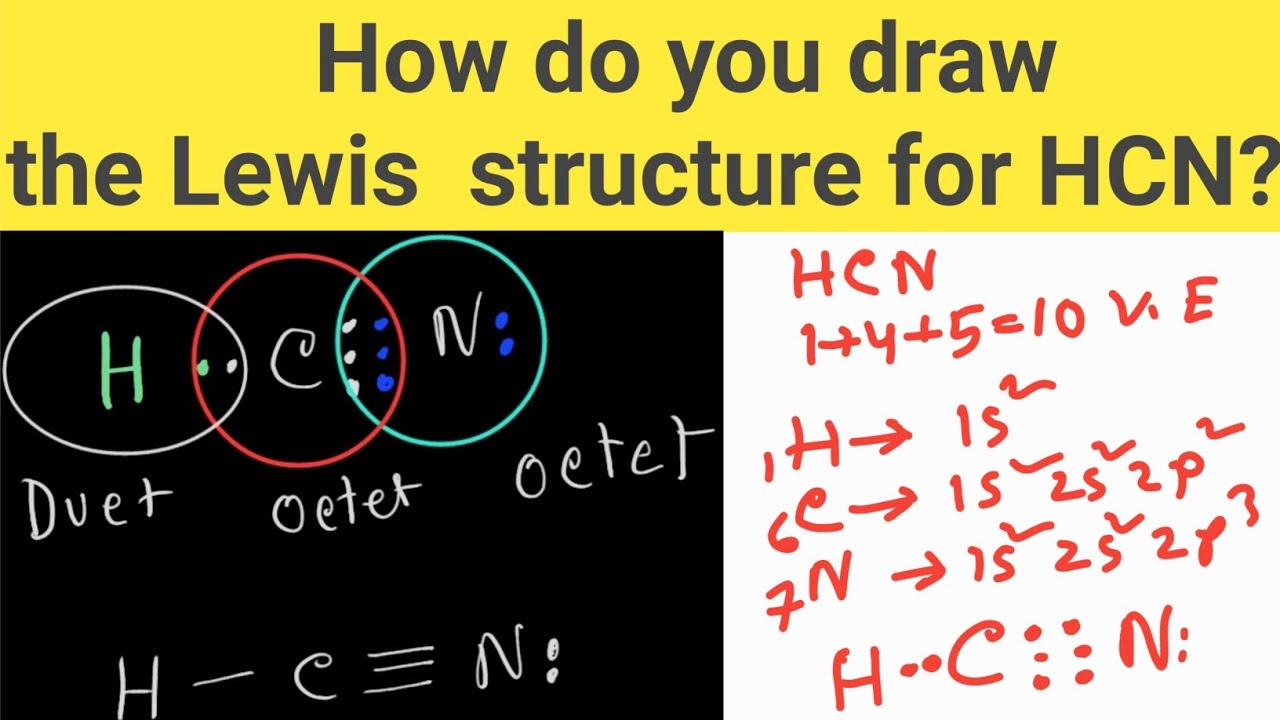
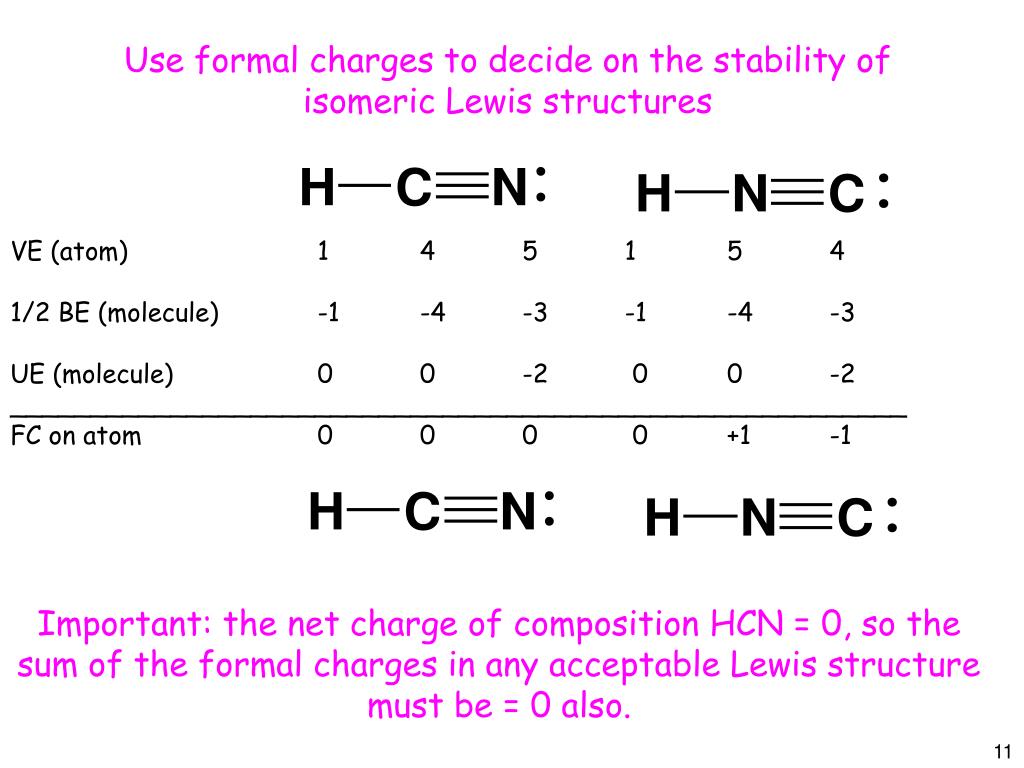
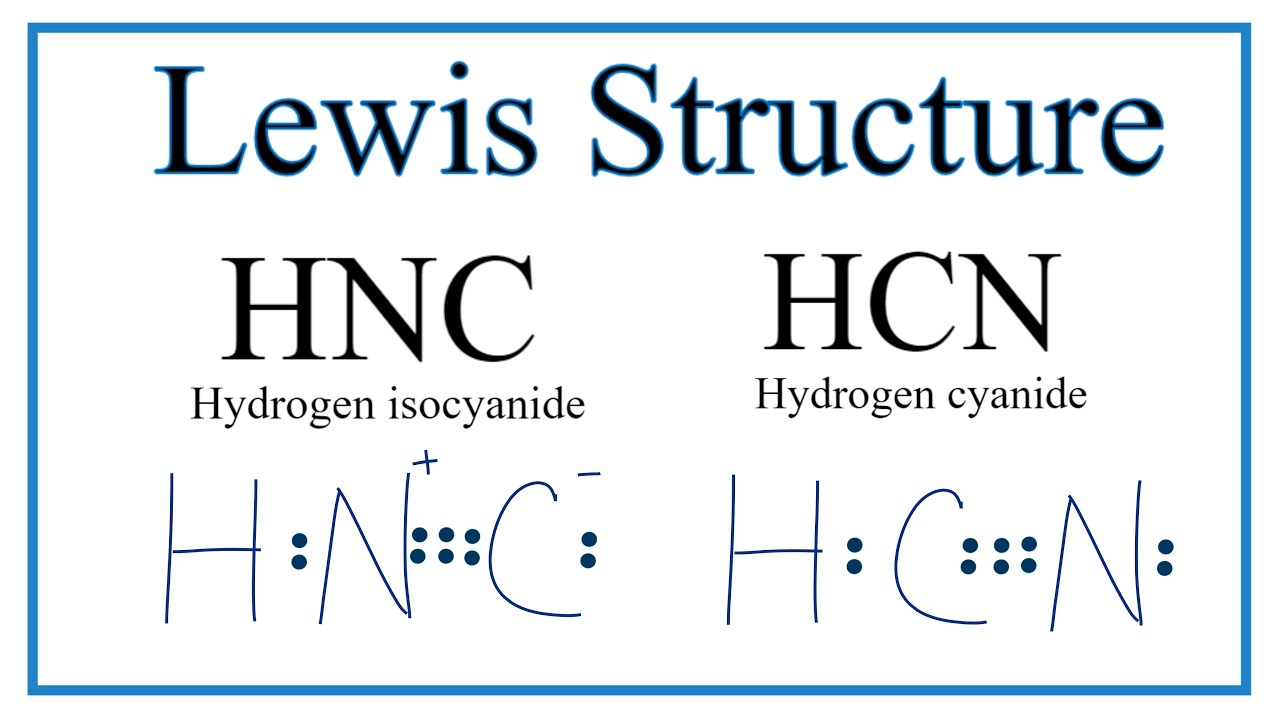
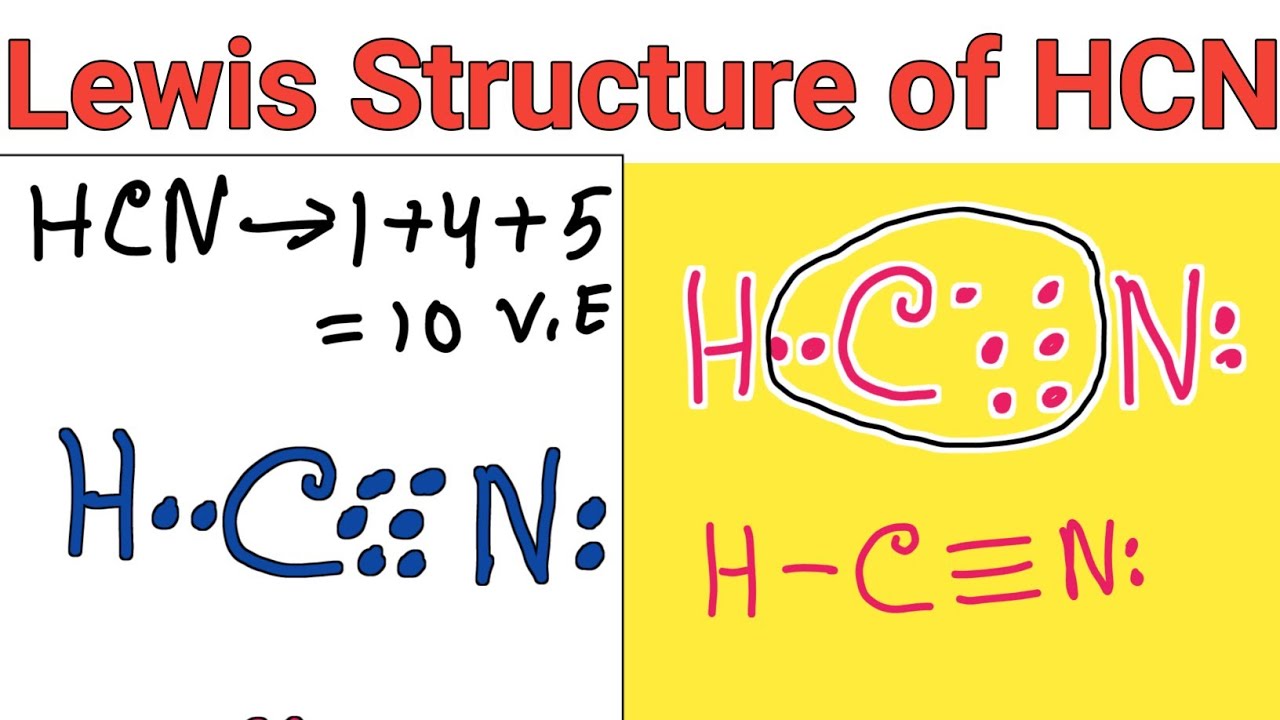
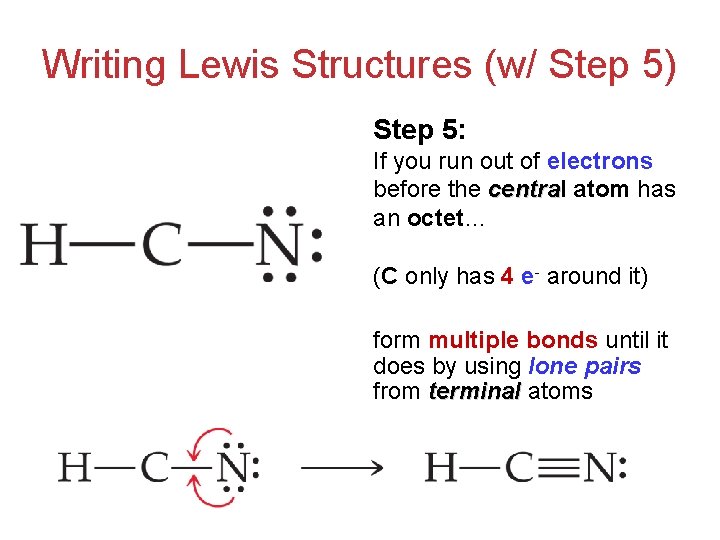
Draw The Lewis Structure Of Hcn - Hcn is a linear molecule, since it has only two electron groups and there are no lone pairs on the central carbon atom. The molecule is made up of one hydrogen atom, one carbon atom and a nitrogen atom. For hcn, hydrogen has one valence electron, carbon has four valence electrons, and nitrogen has five valence electrons, making a total of ten valence electrons. Count the valence electrons you can use. Pcl 3 has 5 valence electros in p and 7 in each of the three cl: There is also a video and a study guide to help with other lewis dot problems. = 5 + 7x3 = 26 valence electrons Web we show two methods to find correct lewis structure of hcn. Web 6 steps to draw the lewis structure of hcn step #1: Put least electronegative atom in centre3. Web in this example problem, we draw the lewis structure for hcn, which has a triple bond. Some compounds have a very unique and different lewis structure and hcn is one of those. Count the valence electrons you can use. Ch 4 has 4 valence electrons in c, and 1 in each of the four h: Here's how to do it. Put one electron pair in each bond4. Carbon has 4 valence electrons, while each oxygen atom has 6. Pcl 3 has 5 valence electros in p and 7 in each of the three cl: One uses math, the other puzzle pieces to give the three correct structure. Web drawing the lewis structure for hcn. Web 6 steps to draw the lewis structure of hcn step #1: Web in this video, we will look at the lewis structure of hydrogen cyanide having a chemical formula of hcn. Web we show two methods to find correct lewis structure of hcn. Web the lewis structure (lewis dot diagram) for hcn.1. Here's how to do it. Web we show two methods to find correct lewis structure of hcn. = 4 + 1x4 = 8 valence electrons; Carbon has 4 valence electrons, while each oxygen atom has 6. One uses math, the other puzzle pieces to give the three correct structure. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion. Make sure you put the correct atom at the center of the hcn molecule. Web use these steps to correctly draw the hcn lewis structure: Determine the total number of valence electrons by adding the valence electrons of each atom in the molecule. The associated charge balances the 7 protons in the nitrogen nucleus, so the nitrogen is formally neutral.. Here's how to do it. In order to draw the lewis structure of hcn, first of all you have to find the total number of valence electrons present in the hcn molecule. Describe the interactions between atoms using lewis structures (what happens to the valence electrons) Web we show two methods to find correct lewis structure of hcn. Web to. The associated charge balances the 7 protons in the nitrogen nucleus, so the nitrogen is formally neutral. Make sure you put the correct atom at the center of the hcn molecule. Count the valence electrons you can use. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3 calculate and mark formal charges on the atoms,. Web in this video, we will look at the lewis structure of hydrogen cyanide having a chemical formula of hcn. Web hcn lewis structure: The hydrogen atom is bonded to the carbon atom, and the nitrogen atom is bonded to the carbon atom via a triple bond. The associated charge balances the 7 protons in the nitrogen nucleus, so the. How to draw the lewis structure for hcn. The hydrogen atom is bonded to the carbon atom, and the nitrogen atom is bonded to the carbon atom via a triple bond. Determine the total number of valence electrons by adding the valence electrons of each atom in the molecule. Web draw the lewis structures of ch 4, pcl 3, co. The molecule is made up of one hydrogen atom, one carbon atom and a nitrogen atom. Be sure that you don't use more than the ten valence electrons available. Web in this example problem, we draw the lewis structure for hcn, which has a triple bond. #1 first draw a rough sketch #2 mark lone pairs on the atoms #3. Web draw the lewis structures of ch 4, pcl 3, co 2, and hcn. Web the lewis structure (lewis dot diagram) for hcn.1. Web to draw the hcn lewis structure, follow these steps: Web the lewis structure of hcn shows that the carbon atom is the central atom and is covalently bonded to both hydrogen and nitrogen atoms. Draw lewis. The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Put least electronegative atom in centre3. Put one electron pair in each bond4. Web the lewis structure (lewis dot diagram) for hcn.1. Web the lewis structure of hcn shows that the carbon atom is the central atom and is. Web the nitrogen nucleus has 3 electrons from the triple bond, and 2 electrons from its lone pair, and 2 inner core electrons; H + c + n =1 + 4 + 5 = 10. Describe the interactions between atoms using lewis structures (what happens to the valence electrons) Web lewis structure of hcn. Web we show two methods to find correct lewis structure of hcn. Add these electrons to give every atom an octet. Here's how to do it. Web use these steps to correctly draw the hcn lewis structure: Web we show two methods to find correct lewis structure of hcn. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Lewis structures can also be useful in predicting molecular geometry in. The carbon atom has (or shares) 3 electrons from the triple bond, and a lone pair of electrons, which it owns. Some compounds have a very unique and different lewis structure and hcn is one of those. Make sure you put the correct atom at the center of the hcn molecule. There is also a video and a study guide to help with other lewis dot problems. Put least electronegative atom in centre3.Draw The Lewis Dot Structure Of Hydrogen Cyanide Hcn vrogue.co
Lewis Structure Of Hcn
Estrutura De Lewis Hcn
Hcn Lewis Structure Bonds Draw Easy
HCN Lewis StructureHydrogen Cyanide (HCN) Lewis Dot StructureDraw
Lewis structure of HCN (Hydrogen cyanide) YouTube
Draw the Lewis dot structure of Hydrogen cyanide (HCN) molecule
HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and
Hcn Lewis Structure Bonds Draw Easy
Estrutura De Lewis Hcn ENSINO
One Uses Math, The Other Puzzle Pieces To Give The Three Correct Structure.
Web Hcn Lewis Structure:
In Order To Draw The Lewis Structure Of Hcn, First Of All You Have To Find The Total Number Of Valence Electrons Present In The Hcn Molecule.
Put The Least Electronegative Atom C In The Middle With H And Cl On Either Side.
Related Post: